Gluetacs jointly reported the mechanism of SOS1 degrader overcoming KRAS mutation and BCR-ABL-positive CML resistance in Cancer Research
ON:2024-11-1 TAGS:GLUETACS THERAPEUTICS
Recently, Gluetacs collaborated with the team of Jiang Biao from ShanghaiTech University and the team of Xie Chengying from Lingang Laboratory to publish a research paper titled “Targeted Degradation of SOS1 Exhibits Potent Anticancer Activity and Overcomes Resistance in KRAS-Mutant Tumors and BCR-ABL-Positive Leukemia” in the internationally renowned journal Cancer Research. The study reported a novel PROTAC degrader of the SOS1 (Son of sevenless homolog 1) protein, SIAIS562055, which significantly inhibits tumor growth by blocking the kinase activity of KRAS and RAC proteins. When used in combination with KRAS inhibitors or ABL inhibitors, it demonstrates excellent synergistic effects and overcome resistance in KRAS-mutated tumors or BCR-ABL-positive chronic myeloid leukemia (CML).

Among all known oncogenes, KRAS is one of the genes with the highest mutation frequency, and its mutations are closely related to the development of a series of refractory cancers, such as non-small cell lung cancer, pancreatic cancer, and colorectal cancer. SOS1 is a guanine nucleotide exchange factor (GEF) that regulates the exchange of guanosine triphosphate (GTP) and guanosine diphosphate (GDP) on small GTP-binding proteins (GTPases) such as RAS and RAC, thereby activating downstream signaling pathways. In theory, inhibiting SOS1 can effectively block KRAS signaling, thus achieving broad inhibition of different types of KRAS mutations. Moreover, studies have shown that BCR-ABL drives the activation of downstream RAS, RAC, and other GTPases signals by recruiting SOS1 to form a complex, participating in the development of blood tumors such as BCR-ABL-driven CML. Therefore, SOS1 may be a potential target for the treatment of CML. To date, no SOS1 inhibitors have been approved for marketing, and several SOS1 inhibitors are undergoing clinical studies aimed at treating KRAS-mutated tumors. However, small molecule SOS1 inhibitors face issues such as pharmacokinetics, safety, and single-use efficacy in clinical studies, and their potential as a strategy for treating CML has not been fully explored. Therefore, there is an urgent need to develop new strategies to effectively inhibit or degrade SOS1 and expand the indications for targeted SOS1 therapy.
The research team synthesized a series of SOS1 PROTAC molecules based on analogs of the small molecule SOS1 inhibitor BI-3406, CRBN ligands, and various types of linkers. After molecular, cellular, and pharmacokinetic studies, SIAIS562055 was selected as the most potent PROTAC, which has high degradation activity against SOS1. This compound can continuously reduce SOS1 levels and inhibit downstream signaling, showing superior antitumor activity in KRAS-mutated tumors and BCR-ABL-positive CML compared to the warhead. Further studies have shown that in KRAS-mutated tumors and acquired resistance models against KRAS inhibitors, SIAIS562055 exhibits significant synergistic antitumor activity in combination with KRAS inhibitors, further inhibiting the ERK signaling pathway, inducing tumor regression, and overcoming resistance. In BCR-ABL-positive CML, SIAIS562055 upregulates the organic cation transporter protein SLC22A4, promoting the active uptake of BCR-ABL inhibitors. When used in combination with BCR-ABL inhibitors, it synergistically enhances the inhibition of ABL phosphorylation and downstream signaling, showing strong antitumor activity in mouse xenograft models and primary CML patient samples without significant toxicity.
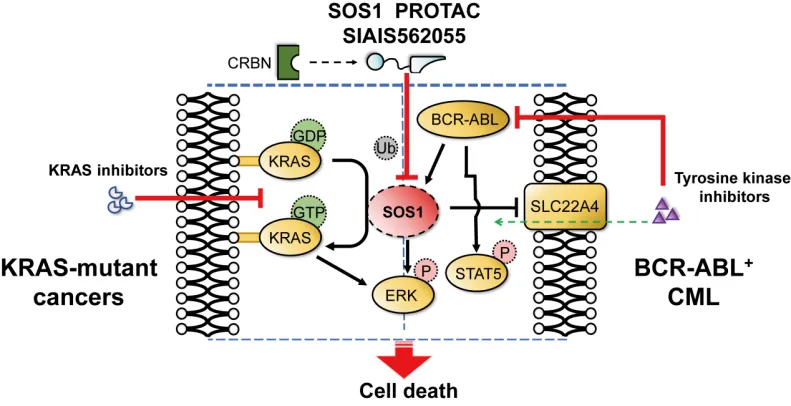
In summary, the novel SOS1 PROTAC SIAIS562055 can effectively reduce the activity of downstream signaling pathways of SOS1, significantly inhibit the growth of KRAS-mutated and BCR-ABL-positive tumors in vitro and in vivo, and exert synergistic antitumor effects with KRAS inhibitors or BCR-ABL inhibitors to overcome resistance. Additionally, this study expands the scope of application of SOS1 and provides important experimental evidence for overcoming resistance to KRAS and BCR-ABL inhibitors.
Ph.D. student Luo Ziwei and master student Lin Chencen from Shanghai Institute for Advanced Immunochemical Studies of ShanghaiTech University are the co-first authors of this paper. Researcher Xie Chengying from Lingang Laboratory, Distinguished Prof. Jiang Biao from Shanghai Institute for Advanced Immunochemical Studies of ShanghaiTech University, and Dr. Yang Xiaobao from Gluetacs are the co-corresponding authors of this paper. This project was funded by Lingang Laboratory (LG202101-01-06) and the National Key Research and Development Program (2022YFC3401500). Additionally, this project received significant support from ShanghaiTech University, the Instrument Platform of Shanghai Institute for Advanced Immunochemical Studies at ShanghaiTech University, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai Jiao Tong University School of Medicine Affiliated Shanghai General Hospital, and Shuguang Hospital affiliated to Shanghai University of Traditional Chinese Medicine.